Panaxosides are effective saponins typical of ginseng ( Panax ). Panaxosides are glycosides with triterpenoid (steroidal) aglycone and one or more sugar residues. With all its vagueness, the word saponin suggests that panaxosides are amphiphilic and therefore able to penetrate the gastrointestinal tract into blood, brain, and all cells. Ginsenosides are a subset of panaxosides (often with all panaxosides incorrectly called ginsenosides ). Panaxosides have extensive modulation effects on physiological pathways ( Attele1999gpm , Christensen2009gcb ), are harmless and are the essence of the adaptogenic effect of ginseng.
Panaxosides act as membrane and nuclear receptors Panaxosides are amphiphilic glycosides Aglycones are divided into damars ( diol and triol ) and oleanic acids, neuro steroid and hormonal modulators Like pancreatic hormones, panaxosides penetrate into the bloodstream, brain and cells, where they act mainly in the nucleus by changing gene expression, influencing a number of genes, such as regulators of inflammation and nitric oxide (NO) synthesis - see, for example, glucocorticoid receptors and PPAR receptors, and their cellular membrane stabilization ( Li1997eag , Choo2003aag )
Main ginseng panaxosides
In 2008, 182 different Panaxosides were isolated from the Panax family (see Christensen2009gcb ), and others are slowly growing. Of these, at least 50 are found in the right ginseng. We are interested in the panaxoside root, which is remarkable by their high weight content: Panaxosides are formed by the age and quality of 2-20% of the dry weight of the root ( Zhu2004cst , Christensen2009gcb , Yuan2002gva ). Specific panaxoside profiles also include leaves, flowers and red ginseng berries. The main panaxosides are:
| Panaxosid | effect |
|---|---|
| ginsenoside Rb 1 | protects memory and brain, anti-stress, anti-inflammatory, antidiabetic, protects blood vessels from atherosclerosis, suppresses angiogenesis |
| ginsenoside Rb 2 | anti-stress, anti-inflammatory, protects the brain, protects against radiation |
| ginsenoside Rc | anti-stress, anti-inflammatory, strongly protects against radiation |
| ginsenoside Rd | anti-stress, anti-inflammatory, protects the brain, protects blood vessels from atherosclerosis, protects against radiation |
| ginsenoside Rg 3 | strongly releases blood vessels and reduces blood pressure, anti-stress, anti-inflammatory, inhibits NMDA receptors in the hippocampus, neurosteroid effects on membrane receptors in the brain, inhibits fat cell growth, protects against arthritis |
| ginsenoside Rh 2 | restores immunity after radiotherapy and chemotherapy, anti-inflammatory, anti-allergic, inhibition of NMDA receptors in the hippocampus |
| Panaxosid | effect |
|---|---|
| ginsenoside Re | antioxidant, antihypertensive, protects memory and brain, strongly restores immunity after irradiation, protects heart and blood vessels |
| ginsenoside Rf | blocks N-type calcium channels in the brain, regulates fat metabolism by acting on PPAR receptors |
| ginsenoside Rg 1 | anti-inflammatory, antihypertensive, protects the brain, strongly protects against radiation, increases angiogenesis |
| ginsenoside Rg 2 | protects neurons, protects brain against ischemia, protects blood vessels from atherosclerosis, anti-stress, nootropic |
| ginsenoside Rh 1 | anti-inflammatory, anti-allergic, affects estrogen receptors |
How panaxosides work
Panaxosides are amphiphilic - soluble in water and in fats. The bearer of the nonpolar character is the triterpenoid (steroidal) skeleton - aglycon. The carrier of the polar character is oxygen and sugar residues bound to the aglycon. Thus, panaxosides pass into the bloodstream and act - like steroid hormones that resemble their amphiphilic character - both in the nucleus of cells and as a result of neurosteroidal effects on membrane receptors mainly in the brain.
As with steroid hormones, small differences in the triterpenoid nucleus ligands have a great effect on the effect of the molecule, and in panaxosides small differences in sugar residues can be their functional diversity. Unlike steroid hormones, however, panaxosides undergo gradual deglycosylation, which begins in the digestive tract. By deglycosylation, more complex panaxosides become simpler. The simplest panaxosides are the aglycone alone: protopanaxadiol (PPD) , protopanaxatriol (PPT) and others. Even ginseng aglycones have extensive effects on the brain, central-vascular system, potency and other physiological parameters.
Structure of panaxosides
Triterpenoid saponins of ginseng - panaxosides - are divided by the type of aglycon into two groups: damaran and oleanic.
What does "triterpenoid"
The spooky word "triterpenoid" means literally "of the six units of isoprene consisting of". The triterpenoid molecule is simply produced in a cell by combining six units of isoprene:
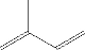
per molecule of squalene:
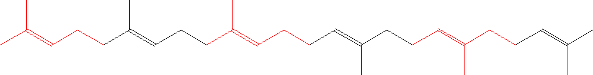
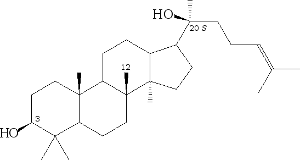
After that, the ginseng cell is made up of either damarendiol:
or beta-amyrin:
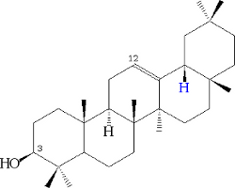
From damarendiol, damaran panaxosides, from β-amyrin oleanate, are also produced.
The synthesis of panaxosides is closely related to human steroid synthesis
The way ginseng produces panaxoside aglycones is very similar to the way our body produces steroid hormones. The difference occurs when rolling the squalene from which our body creates lanosterol:
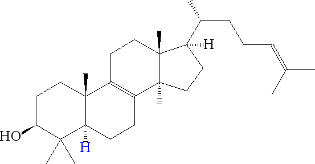
and in about ten steps (see the KEGG database ) cholesterol:
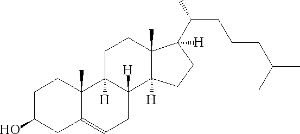
Cholesterol is a substance that is extremely necessary - it serves as a basic raw material for the production of all steroid hormones.
Aglycans of panaxosides
Panaxosides are divided into two main groups according to the type of aglycon: Damaran and oleanic. Damaran panaxosides are further divided into protopanaxadiol (PPD), protopanaxatriol (PPT) and octylol, depending on the location of the sugar residues. Oleanan panaxosides are derived from another aglycon-oleanolic acid. Other types of panaxosides are panaxatriol and damarndiol. The four malonyl derivatives of panaxosides Rb 1 , Rb 2 , Rc and Rd together with panaxoside Ro and other esterified panaxosides are called "acid panaxosides", while others are called "neutral panaxosides" ( Christensen2009gcb , Attele1999gpm ).
Note: The word "damaran" comes from the white damaron ( Agathis dammara , beehive, see the database of angiosperms ), from which damar resin is obtained for the production of varnishes. The word "oleanic" comes from the name of the olives ( Olea ). The origin of the word "okotilol" is unknown.
Panaxadioly - general structure and examples of representatives:
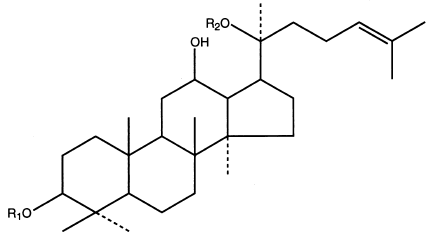
| Panaxosid | R1 | R2 |
|---|---|---|
| Rb1 | -glc (2-1) glc | -glc (6-1) glc |
| Rb2 | -glc (2-1) glc | -glc (6-1) arap |
| Rc | -glc (2-1) glc | -glc (6-1) araf |
| Rd | -glc (2-1) glc | -glc |
| Rg3 | -glc (2-1) glc | -H |
| Rh2 | -glc | -H |
Panaxatrioly - general structure and examples of representatives:
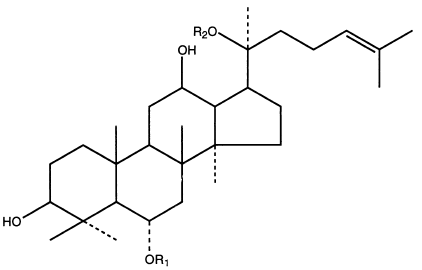
| Panaxosid | R1 | R2 |
|---|---|---|
| Re | -glc (2-1) rha | -glc |
| Rf | -glc (2-1) glc | -H |
| Rg1 | -glc | -glc |
| Rg2 | -glc (2-1) rha | -H |
| Rh1 | -glc | -H |
We can notice that the differences between the panaxosides described above are minimal from the viewpoint of the human observer. Less typical panaxosides are:
Panaxosid Rh3:
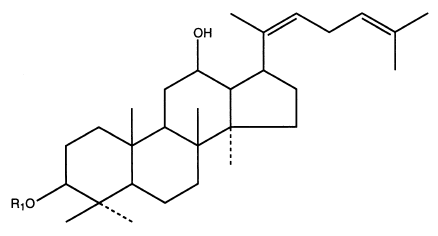
The general structure of oleic acid esters:
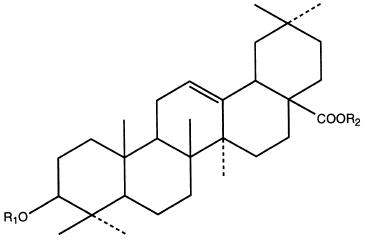
eg panaxoside Ro: R1 = -glcUA (2-1); R2 = -glc
General properties of panaxosides
The panaxoside structure has their typical chemical properties:
- Panaxosides are chemically stable. In addition to one unsaturated C = C linkage, the steroidal panaxoside skeleton contains only aliphatic linkages whose stability is generally known. I was surprised, however, by the recent reading that the glycosidic bond (via oxygen) that the sugars are bound to is also amazingly stable - under standard conditions, its hydrolysis half life is incredible 20 million years, about 100 times more stable than the phosphodiester binding of the DNA strand and 100,000 times more stable than the peptide bond of proteins ( Wolfenden 19988g ).
- Panaxosides are amphiphilic . The steroidal skeleton is hydrophobic with affinity to fatty substances, while sugar residues in panaxosides confer water solubility. This is also associated with their further feature:
- Panaxosides are highly mobile. The ability of ginsenosides to move both in the aqueous and fatty environment helps them to easily enter the human body, including the blood-brain barrier.
Which panaxosides are most abundant in ginseng?
In terms of quantity, more than 90% of the total panaxoside content of ginseng root consists of the following 10 panaxosides ( Christensen2009gcb ):
- PPD ginsenosides Rb1, Rb2, Rc and Rd
- PPT ginsenosides Re and Rg 1
- acidic ginsenosides malonyl-Rb1, malonyl-Rb2, malonyl-Rc and malonyl-Rd
The amount and content of panaxosides varies greatly according to the age and conditions in which ginseng grew. However, there are panaxoside profile specifics that distinguish between genuine ginseng, American ginseng , notoginsenge and other Panax species .
What are the differences in panaxoside profile of ginseng right and American?
Ginsenoside Rf is characteristic of ginseng and is not present in American ginseng or other Panax species. Octylol panaxoside 24 (R) -pseudoginsenoside F11 is again characteristic of American ginseng, whereas in ginseng right it almost does not occur. These panaxosides can be used to distinguish these two species of ginseng ( Christensen2009gcb ). Another difference is in the ratio of ginsenosides Rb 1 / Rg 1 . The Rb 1 / Rg 1 ratio is greater than 10 in American ginseng but ranges from 1 to 3 in the ginseng. The American ginseng generally contains more PPDs than PPT panaxosides, while ginseng is the reverse. The ratio of malonyl-Rc and malonyl- Rb2 ginsenosides to malonyl-Rb 1 ginsenoside is lower in ginseng than in ginseng.
What are the specifics of panoxoside profile notoginsengu?
The specificity of notoginseng ginseng is the ratio of malonyl-ginsenoside content. The most abundant malonyl-ginsenoside is notoginsengu ginsenoside malonyl-Rb 1 .
Panaxosides, apart from the Panax, are not found anywhere else
The rule for plants is that the secondary metabolites contained in them occur in many different plant species. The ginseng is different - panaxosides are unique and characteristic of the genus Panax . I know of one other science known to the panaxoside plant: The gynostem five-leaf squash contains triterpenoids, nine of which are identical to the known panaxosides ( Christensen2009gcb , page 29, Table 1.1). The Gynostema five-lobster, also known as pchin-jin jiaogulan , improperly "five-leaf ginseng", contains these panaxosides in leaves, and its cultivation represents a cheap source of these saponins for those seniors who are deep in their pockets. The content of panaxosides in gynostemy leaves is about 1.5% dry weight, that is, as in a weak young ginseng.
Changes of panaxoside profile in red ginseng processing
To increase the shelf life and improve some pharmacological properties, ginseng root ( ginseng radix album ) is processed by soaking red ginseng, ginseng radix rubra . Even when ginseng is dried, some acidic panaxoside residues and partial deglycosylation occur. The subsequent ginseng gry, which takes place at ~ 100 ° C and takes several hours, carries the Maillard reaction (caramelization) and continues the deglycosylation of panaxosides. Red ginseng therefore has a higher ratio of simpler panaxosides (eg ginsenoside Rg 3 and compound K ) and also contains more free aglycone (mainly PPD and PPT). Further hydrolysis and deglycosylation of panaxosides occurs in the digestive system.